Write the Complete Electron Configuration for the Iron Atom
Its valence orbitals are the 4s and 3d s. Expert Answer 100 5 ratings Previous question Next question.

Electron Configuration For Iron Fe Fe2 And Fe3
Who are the experts.
. The iron atom exhibits Fe 2 and Fe 3 ions. This problem has been solved. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital.
The next six electrons will go in the 2p orbital. Using NOBLE GAS notation write the electron configuration for the calcium atom. In writing the electron configuration for Iron the first two electrons will go in the 1s orbital.
The atomic number of iron is 26. Using NOBLE GAS notation write the electron configuration for the chromium atom. What is the electron configuration of atomic number 26.
Atomic spectrum A representation of the atomic spectrum of iron. Using NOBLE GAS notation write the electron configuration for the copper atom. The s orbital holds a maximum of 2 electrons.
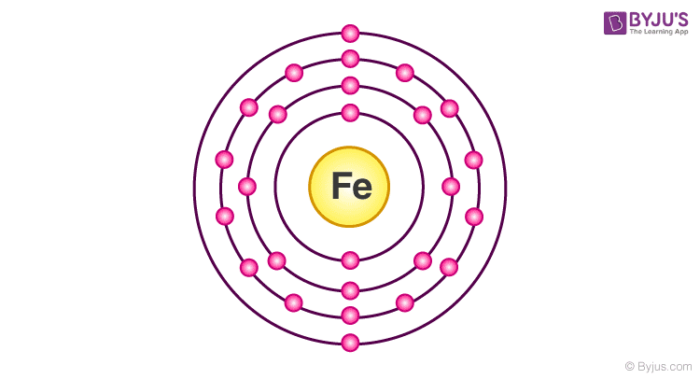
Therefore the next two electrons enter the 2s orbital. The Kossel shell structure of iron. The complete electron configuration of iron contains 26 electrons because 2 2 6 2 6 6 2 122 6 4 s 2.
Schematic electronic configuration of iron. Up to 256 cash back Write the complete ground-state electron configuration for scandium Z21 atom. There are two types of iron ions.
Well put six in the 2p orbital and then put the next two. 1Write the complete electron configuration for the iron atom. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital.
Write both the complete electron-configuration notation and the noble-gas notation for iodine I. Chemistry Electron Configuration Electron Configuration 1 Answer anor277 Jun 18 2018 Well for iron Z 26. The Aufbau electron configuration method is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d.
Write the complete electron configuration for the common monatomic ion formed by the element magnesium Mg. What is the full electron configuration of iron. Using NOBLE GAS notation write the electron configuration for the copper Iion.
Practice Writing the Electron Configuration for an Atom using the Periodic Table with practice problems and explanations. The p orbital can hold 6. The electron configuration of iron atomic number 26 is Ar3d64s2.
The Madelung rule gives the order. The s-orbital can have a maximum of two electrons. And if there are 26 positively charged particles in the NEUTRAL atomthere MUST be 26 negatively charged particles WHIZZING round this nuclear core.
Get instant feedback extra help and step-by-step explanations. Electron Configuration of Oxygen. Its core orbitals are the 1s 2s 2p s 3s and 3p s.
Answered by Raquel Becker on Tue Sep 7 2021 351 AM. Use a noble gas core to write the electron configuration of iron. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2.
The p-orbital can have a maximum of six electrons. Write the complete electron configuration for the oxygen atom. The expanded electron configuration is 1s22s22p63s23p63d64s2.
Expert Answer 100 1 rating Transcribed image text. K shell 2 electrons L shell 6 electrons Therefore the electron configuration of oxygen is 1s22s22p4 as shown in the illustration provided below. Write the complete electron configuration for the iron IIion.
Therefore the valence electrons of iron are eight. Write the complete electron configuration for the iron atom. 2Write the complete electron configuration for the iron atom.
Of the elements discussed in the video iron is most like Sc but iron has 5 more electrons in the last subshell which is filled with electrons making its configuration end in 3d6 rather than 3d1 Hope this helps. Iron has 26 electrons so its normal electron configuration would be. The outer shell is then 4s2 3d6.
The next six electrons will go in the 2p orbital. The period or row numbers 1 through 7 are the energy levels of the elements. How did I know that.
The electron configuration for Iron or Fe begins with a base state of Ar. This ion is an with a charge of. Iron atoms have 26 electrons and the shell structure is 28142.
In order to write the electron configuration for Iron Fe we first need to know the number of electrons for the Fe atom there are 26 electrons. 1 What is the element with an electron configuration of 1s22s22p63s23p64s23d7. The f orbital can hold 14 electrons.
A Write the complete electron configuration for the iron atom B Using noble gas notation write the electron configuration for the maganese atom. The first two electrons of iodine enter the 1s orbital. The d orbital can hold 10.
When we make a 3 ion for Iron we need to take the electrons from the outermost shell first so that would be the 4s shell NOT the 3d shell. Its electrons are filled in the following order. 4s2 and the term symbol is 5D4.
The p orbital can hold up to six electrons. The p orbital can hold up to six electrons. Using SPECTROSCOPIC notation write the complete electron configuration for the iron atom.
A Write the complete electron configuration for the iron atom B Using noble gas notation write the electron configuration for the maganese atom. The ground state electron configuration of ground state gaseous neutral iron is Ar. Using NOBLE GAS notation write the electron configuration for the manganese atom.
Electron Configuration of Oxygen The atomic number of oxygen is 8 implying that an oxygen atom holds 8 electrons. This electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons. Writing the electron configuration you really only need the valence orbitals and you can omit the core orbitals by notating it via the noble gas shortcut.
But the orbitals overlap. What element has the electron configuration 1s22s22p63s23p64s23d6. One other note on writing electron configurations.
Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. So Ar can be written instead of 1s22s22p63s23p6.
Solved H 2a B Write The Complete Electron Configuration For Chegg Com

Electronic Configuration Of Iron Fe Element Iron Atomic Number
Electron Configuration For Iron Fe Fe2 And Fe3
Solved 8a H 2a 3a 4a Sa 6a Ta He Li Be Write The Complete Chegg Com
No comments for "Write the Complete Electron Configuration for the Iron Atom"
Post a Comment